- Agilent Technologies
- Alltech
- AppliChrom
- Avantor (ACE, HiChrom, Ultrasphere)
- Benson Polymeric
- BGB Analytik
- Bischoff Chromatography (ProntoSIL, HIPAK, Enviro, PolyEncap)
- Chiral Technologies (Daicel)
- ChromaNik
- Concise Separations
- Dikma Technologies
- Eurospher
- Exsil (Exmere)
- Fortis Technologies
- GL Sciences
- GRACE
- GROM
- HALO (AMT)
- Hamilton
- HELIX Chromatography
- Jones Chromatography
- Kromasil
- L-column (CERI)
- LiChrosorb
- LiChrospher
- Nacalai Tesque (COSMOSIL, COSMOSCORE, COSMOGEL)
- Nova-Pak
- Ohio Valley
- Optimize Technologies
- Osaka Soda (Shiseido)
- Princeton Chromatography
- REGIS Technologies
- Restek
- Sepax Technologies
- Shine Ion Chromatography
- Shodex (Showa Denko)
- SIELC Technologies
- Spherisorb
- Sumichiral
- Sumipax
- Superspher
- Thermo Scientific
- Trajan (SGE)
- UCT (Selectra)
- VDS optilab
- Vydac
- Welch
- ZirChrom
Whelk-O1 Series

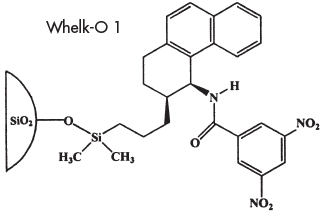
The Whelk-O 1 Chiral Stationary Phase is based on 1-(3,5-Dinitrobenzamido)-1,2,3,4,-tetrahydrophenanthrene. This phase allows separation of nderivatized racemates from a number of families including amides, epoxides, esters, ureas, carbamates, ethers, aziridines, phosphonates, aldehydes, ketones, carboxylic acids, and alcohols. The Whelk-O 1 was originally designed for the separation of underivatized nonsteroidal anti-inflammatory drugs (NSAIDs). This π-electron acceptor/π-electron donor phase exhibits an extraordinary degree of generality, allowing resolution of a wide variety of underivatized racemates. This broad versatility observed on the Whelk-O 1 column, compares favorably with polysaccharide-derived chiral stationary phases. In addition, because of the Whelk-O 1’s covalent nature, this chiral phase is compatible with all commonly used mobile phases, including aqueous systems-a distinct advantage over polysaccharide-derived chiral stationary phases. Other advantages include column durability, excellent efficiency, elution order inversion allowing availability of both enantiomeric forms, and excellent preparative capacity. |
|

